[:The
word petroleum derived from the Latin ‘Petra’ and ‘Oleum’, literally means
"rock oil" and refers to hydrocarbons that occur in sedimentary rocks
of the Earth's crust]
Petroleum distillates is the term
commonly used to refer to aliphatic hydrocarbons. Aliphatic hydrocarbons can
actually be divided into two groups: petroleum distillates and synthetic
paraffinic hydrocarbons. We use petroleum distillates to mean both types of
products.
•Petroleum
distillates include mineral spirits, kerosene, white spirits, naphtha, and Stoddard
solvent. These products may contain trace amounts of benzene and other aromatics.
• When
compared to petroleum distillates, the paraffinic hydrocarbons have lower flammability,
lower aromatic content, narrower boiling range, and higher solvency. They are
also more expensive than the petroleum distillates.
• The
petroleum distillates (and paraffinic hydrocarbons) work well on hard-to clean organic
soils such as heavy oil and ease, tar, and waxes.
• These
products typically have low liquid surface tensions (22 to 28 dynes/cm).This
allows them to penetrate and clean small spaces.
• Petroleum
distillates typically operate at near room temperatures. This is due to the
flammability of the products. However, the flash points may be higher than that
of terpenes.
• Petroleum
distillates are usually used in immersion baths.
• Ultra
Sonic’s may or may not work, depending on the particular product.
• Petroleum
distillates can typically handle high soil loads.
• When the
cleaning power of the bath is exhausted, the entire bath usually needs to be
replaced.
• Petroleum
distillates are compatible with most materials including most elastomers.
Mineral spirits may not be compatible with EPDM, SBR, and Silicone.
• Petroleum
distillates are frequently used in manual wipe-down processes.
• Aliphatic
hydrocarbons are often blends containing oxygenated hydrocarbons.
Flash
points are higher than that of terpenes and traditional solvents. Lower flash points
mean faster drying but more danger of burning.
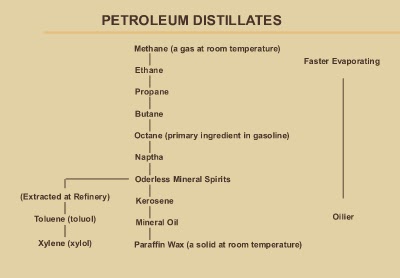
Distillation is the basic process used
to separate and purify the components of crude oil
Anyone making a blanket statement that
products containing petroleum distillates (which include Silicone) are harmful
has no real knowledge of petroleum refining. The advancement of this type of
misinformation is usually meant to take advantage of the consumer's lack of
knowledge in order to sell something that wouldn't sell otherwise, which
reflects the seamier side of negative marketing / advertising.
Petroleum distillates, also called
hydrocarbons or petrochemicals, refer to a broad range of compounds, thick
natural oil obtained from beneath the earth, which are extracted by
distillation during the refining of crude oil. During the fractional distillation
of petroleum, crude oil is heated to (Catalytic cracking) allow various
compounds to turn from liquid into vapour and then captured as they rise, cool,
and condense.
Lighter, more volatile compounds rise
higher before they condense and are collected on distillation trays. Heavier,
less volatile compounds such as diesel fuel and oil are collected on lower
distillation trays. Waxes and asphalts are collected from the bottom after the
other products have volatilized.
Petroleum distillates are found in a wide
variety of consumer-products including lip gloss, liquid gas, fertilizer,
furniture polish, pesticides, plastics, paint thinners, solvents, motor oil,
fuels and hundreds of other products. That a product contains petroleum
distillates does not necessarily make that product harmful but it does depend
upon which petroleum distillate is used and whither it has been further
purified Petroleum distillates listed commonly on labels of general household
products are those that distil off around naphtha’s. Petroleum jelly, a
petroleum distillate product, is generally regarded as non-toxic.
Petroleum distillates contain both
aromatic hydrocarbons (carbon rings) and aliphatic hydrocarbons (straight
carbon chains). The chemical structure of the hydrocarbon largely defines the
nature and behaviour of these compounds.
Distillates
[: a substance that has
been distilled to remove impurities] Distillation
is the basic process used to separate and purify the components of crude oil;
the distilled or purified portion of crude oil usually contains three general
classes of compounds: Aromatic, Naphthenic and Paraffinic Hydrocarbons.
a) Aromatic
hydrocarbons- [:
the term 'aromatic' was assigned before the physical mechanism determining
aromaticity was discovered, and was derived from the fact that many of the
compounds have a sweet scent, the term aromatic in chemistry is no longer
associated with aroma, and many aromatic compounds have no smell] are the most toxic compounds found in petroleum products,
and include such substances as naphthalene, xylene, toluene, and benzene.
Most
aromatic hydrocarbons are long-term toxins and known cancer causing agents,
they are great solvents and a base for many types of useful compounds. The
configuration of six carbon atoms in aromatic compounds is known as a benzene
ring, after the simplest possible such hydrocarbon, benzene. Aromatic
hydrocarbons can be monocyclic or polycyclic. They are a perfect ingredient for
making such things carburettor cleaner or a tar remover where strong solvency
is needed.
b)
Naphthenic hydrocarbons- (aka Cyclo paraffins) after further distillation
aliphatic are used to make Naphthenic oil, a type of mineral oil. In contrast
with paraffinic oils, naphthenic oils contain only low to no proportion of
n-alkanes, being based on cycloalkanes (naphthenes) instead. The
low-temperature behaviour of naphthenic oils is better than of paraffinic oils,
making them suitable for applications that require low pour point. The
degradation products of naphthenic oils are soluble in the oils, leading to
fewer problems with formations of sludge’s and deposits. Naphthenic oils have
different solvent properties than paraffinic oils. Naphthenic oils are
characterized by high proportion of cyclic hydrocarbon fraction. The convention
is that when the paraffinic carbon content is less than 55-60%, the oil is
labelled as naphthenic.
The
principal uses of naphthenic oils are as transformer oils, coolants, solvents,
cutting fluids, and some lubricants, .light oils, solvents and even as a base for
things like detergents and paint dryers and include methane, propane, and
kerosene,
c)
Aliphatic hydrocarbons - the simplest aliphatic compound is methane (CH4). Aliphatic
include alkanes such as fatty acids and paraffin hydrocarbons, alkenes (such as
ethylene) and alkynes (such as acetylene).In organic chemistry, compounds
composed of carbon and hydrogen are divided into two classes: aromatic
compounds, which contain benzene rings or similar rings of atoms, and aliphatic
compounds (G. aleiphar, fat, oil), which do not contain aromatic rings.
In
aliphatic compounds, carbon atoms can be joined together in straight chains,
branched chains, or non-aromatic rings (in which case they are called
alicyclic). They can be joined by single bonds (alkanes), double bonds
(alkenes), or triple bonds (alkynes). Besides hydrogen, other elements can be
bound to the carbon chain, the most common being oxygen, nitrogen, sulphur, and
chlorine. Most aliphatic compounds are flammable, allowing the use of
hydrocarbons as fuel, such as methane in Bunsen burners, and acetylene in
welding.
d) Paraffin
[: the name is derived from the Latin parum (= barely) + affinis with the
meaning here of "lacking affinity", or "lacking reactivity”] -
the simplest paraffin molecule is that of methane, CH4, a gas at room
temperature. Heavier members of the series, such as that of octane C8H18,
appear as liquids at room temperature. The solid forms of paraffin, called
paraffin wax, are from the heaviest molecules from C20H42 to C40H82. Paraffin wax
was identified by Carl Reichenbach in 1830] paraffin compounds have much less
solvency and usually are purified further.
They are used in a myriad of
consumer products, such as a coating for milk cartons and as ingredients in
many lotions and skin creams. Crystal clear white oils are used as a laxative,
to coat pans in bakeries and as a base for medicines. Paraffin wax refers to
the solids with n=20–40. Paraffin compounds are perfect for use as a component
in automotive waxes and polishes and those products used to treat painted
surfaces, vinyl and plastic. Further purification produces Cyclo Paraffin and
it is used in many pharmaceutical and skin beauty products, they are also used
in car care waxes and polishes, they are used as a carrier system as they easily
dissolve wax and provide spread ability and a lubricant for waxes, machine
polishes and glazes.
e) Cyclo
Paraffin - hydrocarbons are used in many car care products and perform many
different and important functions. They are also used in many cleaning products
as solvents to quickly emulsify oils, grease road tar and grime. They will not
harm plastics, vinyl or rubber nor will they remove any important components
like flex agents, plasticizers and etc., while it helps to clean and replace
necessary oils to their surface. Waxes derived from petroleum are much easier
to recover, and offer a wide range of physical properties that can often be
tailored by refining processes.
Most
producers offer two distinct types of petroleum waxes;
1.
Paraffin- [:liquid paraffin has a number of names, including nujol, mineral
spirits, adepsine oil, alboline, glymol, liquid paraffin, medicinal paraffin,
saxol, or USP mineral oil] distinguished by large, well-formed crystals and
micro-crystalline, higher melting waxes with small, irregular crystals. Some
producers also sell "intermediate" wax, the boiling range cut where
the transition in crystal size and structure occurs. Paraffin wax produced from
petroleum is essentially a pure mixture of normal and iso-alkanes without the
esters, acids, etc. found in the animal and vegetable-based waxes. A typical
composition for mineral spirits: aliphatic solvent hexane having a maximum
aromatic hydrocarbon content of 0.1% by volume, is listed as a potential
carcinogen in the MSDS
2. Petroleum
wax - producers also characterize wax by degree of refinement: fully refined
paraffin has oil content generally less than 0.5%, and fully-refined
micro-crystalline less than 1.5%; "slack wax" - precursors to the
fully refined versions in either case would have oil content above 2% and as
high as 35% by weight.
3. Ultra
violet radiation (UVR) protection- The use of petroleum distillates allows
premium ultra violet (UV) radiation absorbers to be included in the formulation
(as an oil-in-water emulsion or by utilizing a resin as its carrier system) as
the most effective ones are not soluble in water. This can provide excellent
protection against deterioration, chalking and fading caused by sunlight for
various plastics, rubber and vinyl dressings. It should be noted that the
protection needs to be renewed periodically as it lessens over time.
Silicone
(Siloxane) oils - are polymers that include silicon together with carbon,
hydrogen, oxygen, and sometimes other chemical elements, which provide an excellent
lubricant that when used as a carrier system in polishes and waxes that makes
them easier to apply and remove When used in paints and other coatings it
ensures an even flow through a spray nozzle ensuring an even product
distribution. It not silicone that you need worry about, just the 'type' (what
it’s formulated with) you need to be aware of. Silicone oils provide an
excellent lubricant that when used as a carrier system in polishes and waxes
that makes them easier to apply and remove When used in paints and other
coatings it ensures an even flow through a spray nozzle ensuring an even
product distribution.
Products
that contain petroleum distillates must be labelled with the phrase, “Contains
petroleum distillates”, regardless of the properties of the distillate used in
its formulation. This labelling is mandated by the Consumer Product Safety
Commission (a federal government agency) this warning is provided to help
doctors and emergency personnel decide how best to treat in gestation. These
warnings have nothing to do with product performance or suitability; the
directions for use and other cautions are for information only
Information resource
1. U.S. Environmental Protection Agency website
2. EPA/Purdue University Study 2001
3. American Association of Industrial Hygiene (AAIH)
4. American Petroleum Institute (API) publications
5. National Petrochemical & Refiners Association (NPRA)
6. U.S. National Library of Medicine - http://householdproducts.nlm.nih.gov/index.htm
I would like to think that these
articles become an asset to anyone who is new to detailing and to professionals
alike, as well as industry experts who seek to advance their knowledge.
I hope the above article was
informative. By having some understanding of the ‘What’ and ‘Why’ as well as
the ‘How’ along with a little science to help you understand how the chemicals
we use react, you can achieve the results you desire.
I would appreciate it if you would share
this article as it helps other detailers further their knowledge.
Questions
and/ or constructive comments are always appreciated
.
Copyright
© 2002 - 2012 TOGWT® (Established 1980) all rights reserved
No comments:
Post a Comment